By definition atoms have no overall electrical charge. Examples of monatomic ions include Na and Cl- etc.
Atomic Number Periodic Table Archives Dynamic Periodic Table Of Elements And Chemistry
1

What Are The First 20 Elements Names And Symbols
The atomic number is the number of protons in an atom of an element.

Atomic number definition. For example if an atom has a Z of 6 it is carbon while a Z of 92 corresponds to uranium. However the nuclear sizes are quite small and need smaller units. By using this chemists work out the chemical formula.
How can you tell one isotope from another. Atomic sizes are on the order of 01 nm 1 Angstrom 10-10 m. Nuclear energies are very high compared to atomic processes and need larger units.
It is the mass of a mole of a substance. Both are abbreviated Z eff. The most commonly used unit is the MeV.
This article will give details of polyatomic ions and their examples. An elements atomic number has a simple definition. This divergence in the graph is due to an increase in the neutron number.
While other numbers may be decimal values the atomic number is always a simple positive whole number. Difference between Atomic Mass and Molar Mass. Atomic structure definition the structure of an atom theoretically consisting of a positively charged nucleus surrounded and neutralized by negatively charged electrons revolving in orbits at varying distances from the nucleus the constitution of the nucleus and the arrangement of the electrons differing with various chemical elements.
The atomic number of carbon is 6 and the atomic number of silver is 47. Atomic number Number of protons. For example in a sodium atom there are 11 electrons and 11 protons.
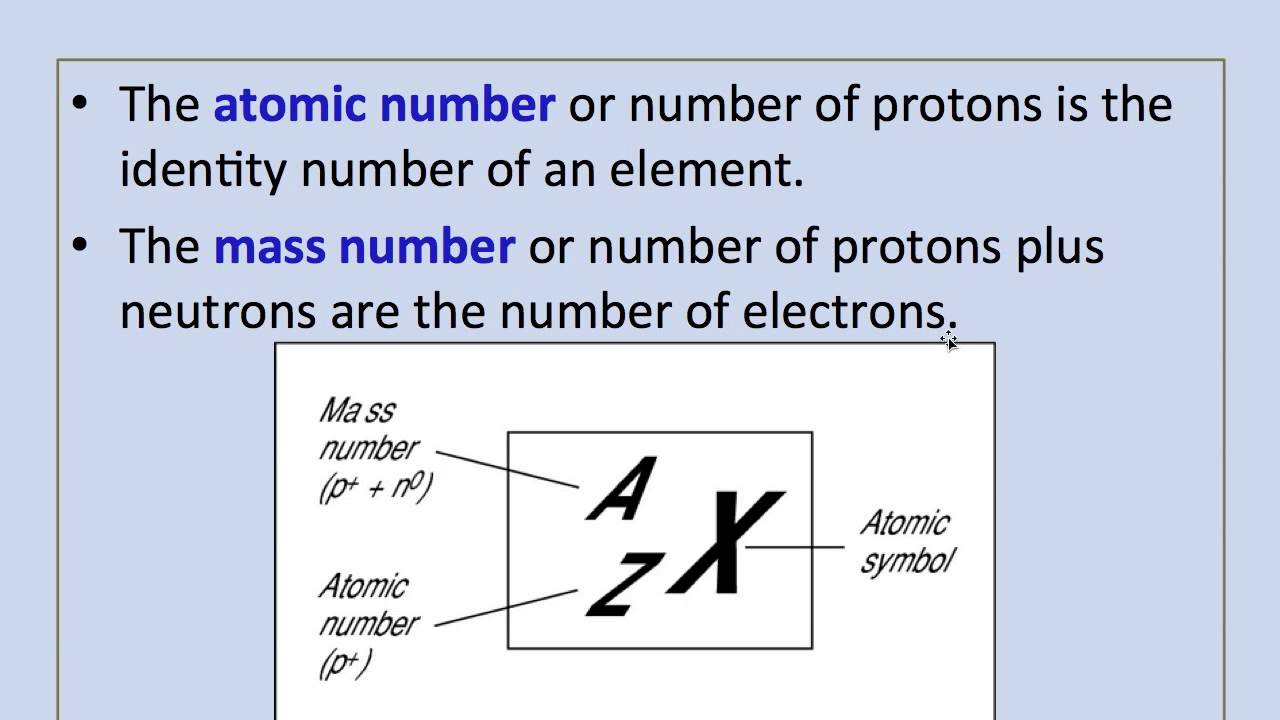
As there are no physical existence of orbital in atoms it is difficult to measure the atomic radius. The single most important characteristic of an atom is its atomic number usually denoted by the letter Z which is defined as the number of units of positive charge protons in the nucleus. See also atomic number cardinal 2 complex number magic number mass number ordinal prime number real number registration number R number serial number whole number Extra Examples Add all the numbers together divide by ten and then take away twelve.
Step 3 - The Number of Electrons is. Atomic Number Orbital Energy Levels. The atomic number of hydrogen is 1.
Thus it is different from monatomic ions which contain only one atom. Thus the atomic number of Na atom number of electrons number of protons 11. 1 GeV 10 9 eV.
It must be added atomism was one of a number of competing theories on the nature of matter. In our example kryptons atomic number is 36. Carbon has an atomic number of six and two stable isotopes with mass numbers of twelve and thirteen respectively.
Of or relating to an atom or atoms. You can find the atomic number from an isotope symbol. The link between the mass of an element and the number of atoms it contains is the relative atomic mass of the element.
The symbol used for it is M. One that is the effective nuclear charge of an atom and one that calculates the average atomic number for a compound or mixture of materials. The atomic numbers go in order on the table.
1 electron volt 1eV 16 x 10-19 joules 1 MeV 10 6 eV. The term poly means many so a polyatomic ion is an ion that contains more than one atom. Use the sim to learn about isotopes and how abundance relates to the average atomic mass of an element.
Effective atomic number has two different meanings. It has a unit of the unified mass unit u or the atomic mass unit amu. Any atom with 47 protons is an atom of silver.
Atomic synonyms atomic pronunciation atomic translation English dictionary definition of atomic. For heavier elements the number of neutrons in the nucleus is more than the number of protons which increases the mass of an atom. Define relative atomic mass.
The number of protons also determines the chemical behavior of the element. Definition of atomic radius The atomic radius is the size of the atom typically measured by the distance from the nucleus of the atom to the electron clouds around the nucleus. The atomic number or proton number symbol Z of a chemical element is the number of protons found in the nucleus of every atom of that element.
Scientists determine the atomic mass by calculating the mean of the mass numbers for its naturally-occurring isotopes. For heavier elements as the atomic number increases the atomic weight exceeds twice the atomic number. The relative atomic mass scale is used to compare the masses of different atoms.
This is the fundamental definition of an element. The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol ZThe number of electrons in an electrically-neutral atom is the same as the atomic number. The total electrical charge of the nucleus is therefore Ze.
It is denoted by m a. The number of protons in one atom of that element. The effective atomic number Z eff sometimes referred to as the effective nuclear charge of an atom is the number of protons that an electron in the element.
Are all atoms of an element the same. The number of protons in an atom is referred to as the atomic number of that element. Atomic number chemical symbol and mass number.
Relative atomic mass synonyms relative atomic mass pronunciation relative atomic mass translation English dictionary definition of relative atomic mass. Accordingly the number of protons which is always equal to the number of electrons in a neutral atom is also the atomic number. Its average atomic mass is 1211.
Understand atomic number. This tells us that an atom of krypton has 36 protons in its nucleus. 1 TeV 10 12 eV.
Atomic theory began as a philosophical concept in ancient Greece and India. Atomic number the number of a chemical element in the periodic system whereby the elements are arranged in order of increasing number of protons in the nucleus. When an electron is at a specific energy level it is more likely to be found in certain portions of that level than others.
For example if you are told the element name is aluminum you can find the name or symbol Al to determine the atomic number is 13. In an uncharged atom the atomic number is also equal to the number of electrons. Varying the number of neutrons in an element changes its isotopes while changing the numbers of electrons makes it an ion.
N the ratio of the average mass per atom of the naturally occurring form of. The atomic number uniquely identifies a chemical elementIt is identical to the charge number of the nucleus. The number of protons determines the total electric charge of the nucleus which determines how many electrons the atom can support.
The meaning of atomic number is an experimentally determined number characteristic of a chemical element that represents the number of protons in the nucleus which in a neutral atom equals the number of electrons outside the nucleus and that determines the. The sum of the atomic number Z and the number of neutrons. These ancient philosophers speculated that the earth was made up of different.
The atomic mass is the sum of the mass of protons neutrons and electrons.

Uranium Definition Properties Uses Facts Britannica

Atomic Number Chemistry For Non Majors

Periodic Table Atomic Number Definition Etc Warm Up In Your Notebooks Write Today S Date The Following Question And The Answer To The Following Ppt Download
Difference Between Atomic Number And Mass Number Definition Explanation With Examples

Atomic Number And Mass Number Structure Of Atoms Cbse Grade 9 Chemistry Youtube
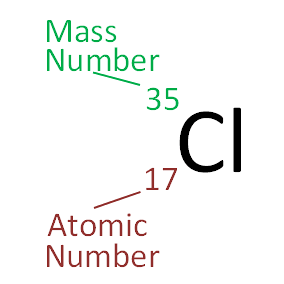
Definition Of Nucleus Chemistry Dictionary
Atomic Number Proton Number Definition Characteristics Nuclear Power Com

Atomic Number Wikipedia

